Banner image: Confluente Rhabdomyosarcoma (RD) cell line under an inverted phase contrast microscope. Credit: Dhifaf zeki, Wikimedia Commons
A guide to Phase Contrast
What is phase contrast? Phase Contrast is a light microscopy technique used to enhance the contrast of images of transparent and colourless specimens. It enables visualisation of cells and cell components that would be difficult to see using an ordinary light microscope.
As phase contrast microscopy does not require cells to be killed, fixed or stained, the technique enables living cells, usually in culture, to be visualised in their natural state. This means biological processes can be seen and recorded at high contrast and specimen detail can be observed. Fluorescence staining can be used in combination with phase contrast to further improve the visualisation of samples.
Phase contrast is ideal for thinner samples, therefore an inverted microscope system can be used. This provides the additional advantage of having more working space. Phase contrast can also be installed on upright microscopes.
If thicker samples need to be visualised in high-resolution, differential interference contrast (DIC) is a more suitable technique to use.
Advantages of phase contrast
- Enhanced Contrast: Allows for the visualisation of transparent and colourless specimens without the need for staining.
- Non-destructive Observation: Enables the observation of living cells in their natural state, as cells do not need to be fixed or stained.
- Real-time Monitoring: Facilitates the real-time study of biological processes within cells and tissues.
- Cost-effective: Does not require expensive dyes or stains, making it more cost-effective for routine laboratory use.
- Ease of Use: Generally simpler to use compared to more complex imaging techniques like fluorescence microscopy.
- Versatility: Suitable for a variety of applications, including the study of microorganisms, thin tissue slices, and subcellular components.
- Combinable: Can be used in conjunction with other techniques like fluorescence staining to further enhance image quality and detail.
Applications of phase contrast
Phase contrast is used to visualise transparent specimens, when high-resolution is not required, including:
- Living cells (usually in culture)
- Microorganisms
- Thin tissue slices
- Fibres
- Subcellular particles, including organelles
What components are needed for phase contrast?
It is relatively simple and inexpensive to adapt an inverted or upright light microscope for phase contrast. The following components need to be installed:
- A phase contrast condenser with a condenser annulus (also known as a phase ring or phase annulus)
- Phase contrast objectives - a set of objectives which each contain a phase plate.
- Phase plate - located in the objective rear focal plane.
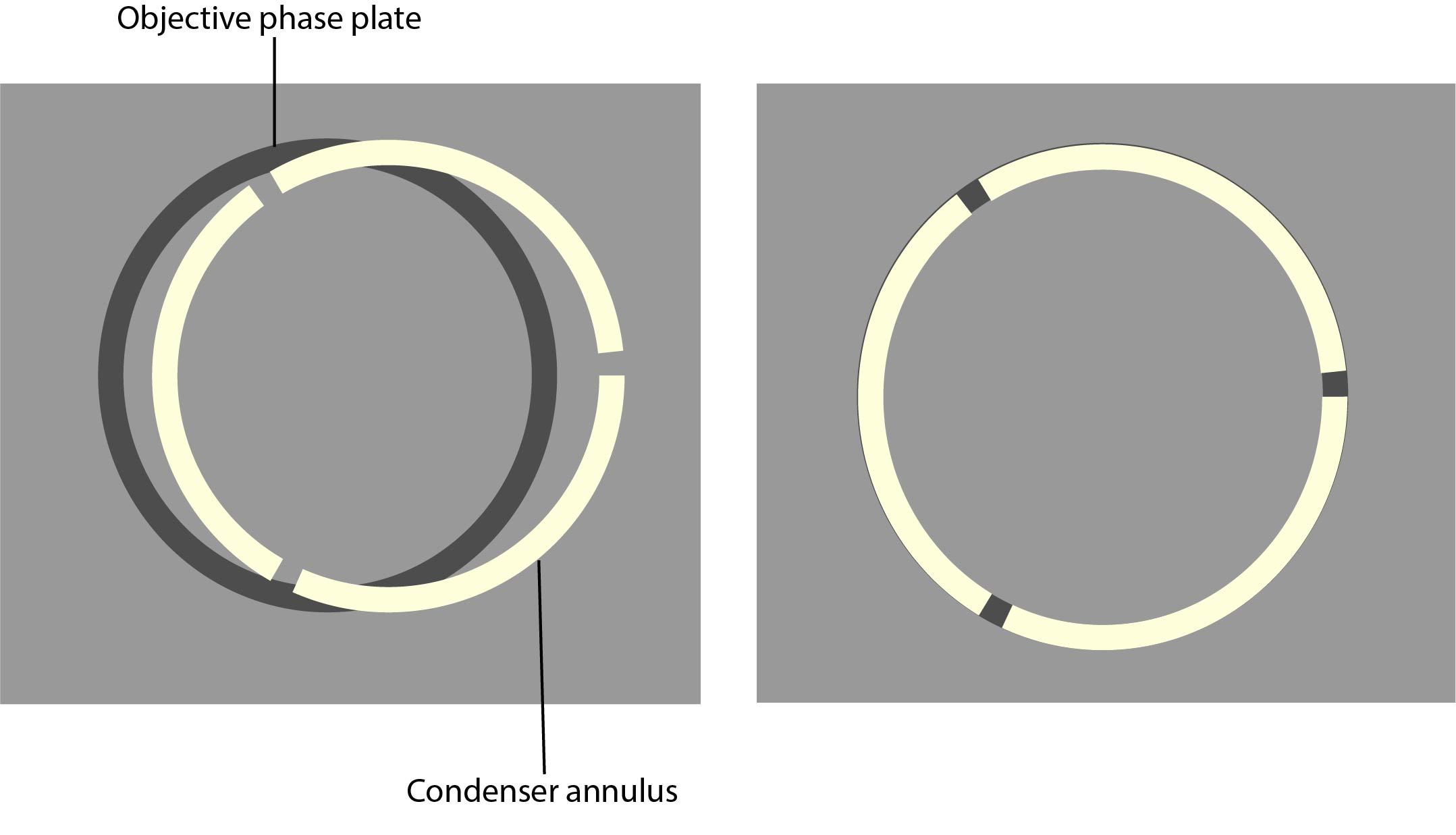
Care must be taken with the condenser annulus and the phase rings, as they need to be matched in diameter and optically conjugated.
How does phase contrast work?
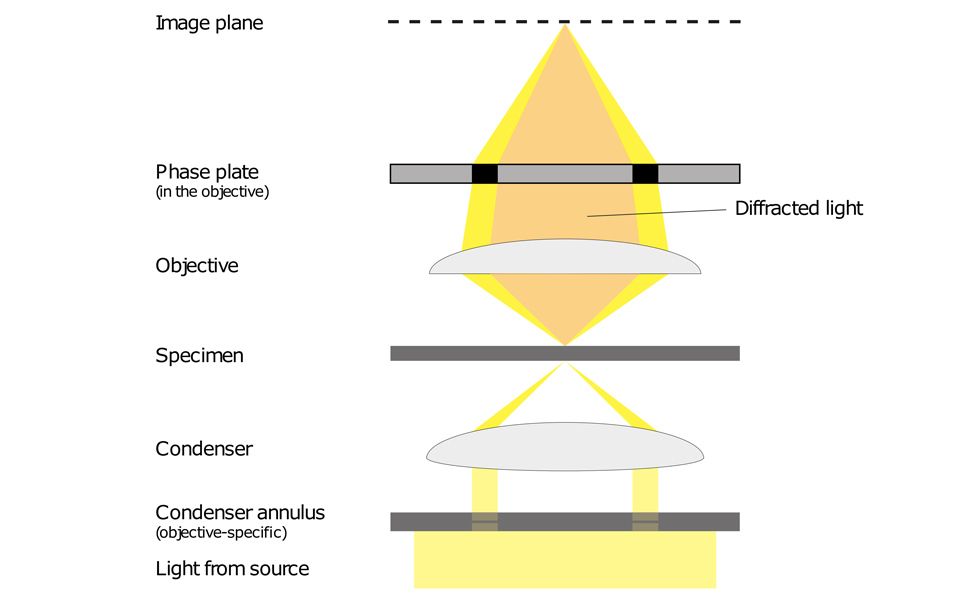
Phase contrast microscopy translates small changes in the phase into changes in amplitude (brightness), which are then seen as differences in image contrast.
Unstained specimens that do not absorb light are known as phase objects. This is because they slightly change the phase of light that is diffracted by them; the light is usually phase-shifted by about ¼ wavelength compared to the background light. Our eyes are unable to detect these slight phase differences as they can only detect variations in the frequency and intensity of light.
Phase contrast enables high contrast images to be produced by further increasing the difference of the light phase. It is this characteristic that enables background light to be separated from specimen diffracted light. The difference of the light phase is increased by slowing down (or advancing) the background light by a ¼ wavelength, with a phase plate just before the image plane. When the light is focused on the image plane, the diffracted and background light cause destructive (or constructive) interference which decreases (or increases) the brightness of the areas that contain the sample, in comparison to the background light.
Light from a tungsten-halogen lamp goes through the condenser annulus in the substage condenser before it reaches the specimen. This allows the specimen to be illuminated by parallel light that has been defocused.
Some of the light that passes through the specimen will not be diffracted (bright yellow in the picture). These light waves form a bright image on the rear aperture of the objective. The light waves that are diffracted by the specimen pass the diffracted plane and focus on the image plane only. This allows the background light and the diffracted light to be separated.
The phase plate then changes the background light’s speed by ¼ wavelength. When the light is focused on the image plane, the diffracted and background light will cause destructive or constructive interference, which changes the brightness of the areas that contain the sample in comparison to the background light. Often the background is also dimmed by 60 to 90% by a grey filter ring.
Aligning your microscope for phase contrast
All of the components required for phase contrast need to be aligned and centred. Some phase sliders are pre-centred, we therefore recommend that you check beforehand. Details of how to centre and align phase components are below:
1. If possible, set up Koehler illumination on your microscope. Read Scientifica’s 6 step guide to Koehler illumination to help you set this up.
2. Install the phase rings in the condenser.
3. Remove the eyepieces and replace these with the phase contrast centring telescope.
4. Put the phase contrast telescope into focus, so that the phase plate and phase ring are in focus.
5. Put the lowest magnification phase objective and corresponding phase annulus in place. For example, a 10x Ph1 objective with a Ph1 phase annulus.
6. Look at the phase plate and phase ring through the phase telescope.
7. Using the adjustment screws on the condenser, centre the phase plate and phase ring so the segmented circle of light sits on the black ring.
8. Repeat steps 4-6 for the rest of the objectives.
9. Once all objectives have been aligned and centred, remove the phase contrast centring telescope and replace this with the eyepieces.
You shouldn’t need to re-centre the phase contrast microscope. It is, however, recommended to regularly check that the set-up is centred using the phase contrast telescope.
All of the components required for phase contrast need to be aligned and centred. Some phase sliders are pre-centred, we therefore recommend that you check beforehand. Details of how to centre and align phase components are below:
Limitations of phase contrast
- Phase contrast images often have halos surrounding the outline of details that have a high phase shift. These halos are optical artefacts and can make it hard to see the boundaries of details.
- The resolution of phase images can be reduced due to the phase annuli limiting the numerical aperture of the system.
- Phase contrast doesn’t work well with thick specimens as these can appear distorted.
Scientifica has created a printable PDF guide, download it here: Phase Contrast

Scientifica PatchScope
An ultra-stable, motorised inverted microscope that can be integrated with your existing manipulators.